Proteins: structure and properties. Functions of proteins
Among complex biopolymers, in terms of functional significance and quantitative ratio, the main role belongs to proteins. In an animal cell they make up 40-50% of its dry mass, in a plant cell - 20-35%. The enormous diversity of living things is largely determined by differences in the composition of their proteins. There are more than 5 million types of them in the human body alone. The reason for such diversity of proteins is explained by the specificity of their structure. Squirrels represent polymers, monomers which are amino acids. There are 20 known types of amino acids that are part of protein molecules. They are called basic in order to distinguish them from other amino acids that are also present in organisms, but are not part of protein molecules. All these amino acids within their molecules have the same atomic structure
H-C-COOH, where COOH is a carboxyl group, and nh 2 is
amino group
The fourth valence bond of carbon is occupied by the so-called radical (R). The radicals of different types of amino acids differ from each other in chemical structure, electrical charges, and also in their ability to dissolve in water. Amino acids are amphoteric compounds, that is, the same amino acid can act as both an acid and an alkali. The carboxyl group (COOH) gives it acidic properties, and the amino group (NH 2) gives it alkaline properties. Due to amphotericity, amino acids can interact with each other, with one of them acting as an acid and the other as an alkali; The carboxyl group of one amino acid interacts with the amino group of another). The connection of amino acids into the protein bud is carried out due to strong covalent bonds. This process can be written using the following equation:
The resulting combination of amino acids is called a peptide, and the covalent bond between them is called a peptide bond. All proteins are polypeptides, i.e. chains consisting of many tens and even hundreds of amino acid units. From 20 types of amino acids you can build a huge number of proteins, just as from 20 letters of the alphabet you can make many words, the meaning of which will depend on what letters they are made of, in what quantity they are taken and in what order they are installed. The properties of a protein molecule are determined by the composition of amino acids, the number of amino acid units, as well as the order in which they appear in the chain.
The sequence of amino acid residues in a protein molecule determines its primary structure (Fig. 1-I). If we consider that the size of one amino acid unit is 0.35-0.37 nm, then it is obvious that a protein macromolecule, which consists of hundreds of amino acid residues, should have a length of several tens of nanometers. In reality, the sizes of proteins are much smaller, because in space the polypeptide chain is completely or partially twisted into a helix, which is secondary protein structure (Fig. 1-II). The amino acid radicals remain outside the helix, and hydrogen bonds are formed between the NH groups located on one turn and the CO groups located on the adjacent turn of the helix. They are much weaker than covalent ones, but, repeated many times, they provide a strong adhesion. A polypeptide helix, “stitched” with numerous hydrogen bonds, is a fairly strong structure. The polypeptide helix undergoes further folding - it folds bizarrely, but for each protein quite definitely and constantly into a so-called globule (ball), which is tertiary structure of the protein molecule (Fig. 1-III).
The tertiary structure of a protein is maintained by three types of bonds: ionic, hydrogen and disulfide, as well as hydrophobic interactions. In quantitative terms, hydrophobic relationships are the most important: in the aqueous environment of the cell, hydrophobic radicals repel each other. Thus, the aqueous environment seems to force the protein molecule to adopt a certain ordered structure, which becomes biologically active.
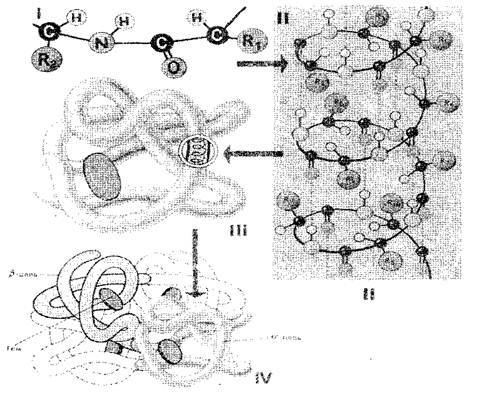
Rice. 1. Scheme of the structure of a protein cell: I, II, III, IV - primary, secondary, tertiary, quaternary structures
Proteins consisting of one polypeptide chain have only tertiary structure. However, some proteins are built from several polypeptide chains. The concept was introduced for them quaternary structure(Fig. 1-IV), which is a single functional unit held together by both hydrophobic interactions and hydrogen and ionic bonds. The quaternary structure is characteristic, for example, of hemoglobin. Its molecule consists of four separate polypeptide chains. Some viruses, such as tobacco mosaic virus, have a protein shell consisting of many polypeptide chains packaged in a highly ordered manner.
Under the influence of various physicochemical factors (the action of concentrated acids and alkalis, heavy metals, high temperature, etc.), the structure, and therefore the properties of protein molecules, can change. The process of disrupting the natural structure of a protein or unfolding a polypeptide chain without breaking peptide bonds is called denaturation squirrel (Fig. 2). As a rule, denaturation is irreversible, however, in the first stages, provided that the action of negative factors is suspended, the protein can restore its original structure - this process is called renaturation squirrel. The process of destruction of the primary structure of a protein is always irreversible; it is called protein destruction.
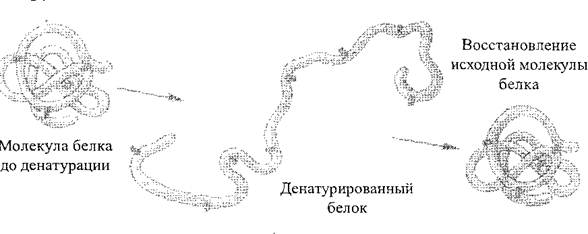
Rice. 2. Scheme of the protein denaturation process
The physical and chemical properties of proteins are very diverse: there are hydrophilic and hydrophobic proteins, some of them easily change their structure as a result of even minor exposure to environmental factors, others are resistant to these factors. Based on their physicochemical properties, proteins are divided into simple (proteins) and complex (proteids).
Simple proteins consist exclusively of amino acid residues, while complex proteins also include compounds of a different nature, such as residues of phosphoric and nucleic acids, carbohydrates, lipids, etc.
The biological functions of proteins are extremely diverse. First of all they do construction function. Proteins are an integral part of cell membranes; they make up such non-membrane cell organelles as microtubules and microfilaments that make up the cell skeleton (cytoskeleton). Cartilage, tendons, and ligaments are made of proteins that have strength and elasticity. Nails and feathers are built from durable and insoluble protein keratin. In addition to construction, squirrels also perform protective a function that consists both in preventing damage to cells, organs and the body as a whole, and in protecting the body from parasites and foreign proteins. In the body of vertebrates, protective proteins are formed - antibodies. These are specialized proteins that are produced by blood lymphocytes. They are able to “recognize” and neutralize bacteria, viruses, and proteins foreign to the body. The blood protein fibrin causes blood clotting, protecting the body from large blood losses.
Regulatory The function of proteins is the ability to regulate metabolic activity with the help of protein hormones, as well as enzyme proteins. Proteins perform and signaling function. It lies in the ability of individual complex proteins that make up the cell membrane to “recognize” specific chemical compounds and react to them in a certain way: bind them, change their structure, transmit signals about the presence of these substances to other parts of the membrane or inside the cell. Thanks to the signaling function of proteins, the cell can selectively absorb substances from the external environment.
Motor the function of proteins is the ability of some of them to contract, thereby providing the ability of a cell, tissue, or organism as a whole to change its shape and move. Thus, due to proteins such as actin and myosin, which are part of muscle cells, the muscle fiber is shortened; The protein turbulin, which makes up microtubules and microfilaments, ensures the movement of cilia and flagella of eukaryotic cells.
Some proteins can be deposited by the cell in reserve, thereby fulfilling storing function.
The endosperm of the seeds of many plant species (wheat, corn, rice) contains proteins that the embryo feeds on in the first stages of its development. The function of these proteins can be defined as nutritious.
Proteins are capable of carrying out transport some substances both inside the cell and inside the body. For example, hemoglobin, a protein in the blood of humans and vertebrates, transports oxygen from the respiratory organs to the cells, and carbon dioxide in the opposite direction.
Energy The function of proteins is that when they are broken down in the cell, energy is released. Some of the amino acids that are formed during breakdown are used for the biosynthesis of new proteins, and the rest are broken down into final breakdown products with the release of energy (with the complete breakdown of 1 g of proteins, an average of 17.2 kJ of energy is released).
One of the main functions of protein is enzymatic. Enzymes are biological catalysts or accelerators of chemical reactions taking place in a living organism. As is known, the rate of chemical reactions significantly depends on the concentration of substances, as well as the temperature of the environment in which these reactions occur. If we consider that the life of a cell (organism) is a combination of a huge number of reactions of synthesis and decay that make up metabolism, then the huge role of protein-enzymes in the vital processes of the organism at all its levels becomes clear. Only thanks to enzymes, at a relatively low temperature of the body and a low concentration of substances in its cells and tissues, chemical reactions proceed at a fairly high speed (an enzymatic reaction proceeds 100-1000 times faster than in a medium without enzymes). The catalytic activity of an enzyme protein is not determined by the entire molecule, but only by a small part of it, called the active center. The spatial structure of the active center, like a key to a lock, matches the shape of the spatial structure of the catalyzed substance (substrate); this is what explains the specificity of enzyme proteins (Fig. 3).
When a protein molecule denatures, the structure of the active center is disrupted and the enzyme loses its catalytic ability. Thus, the protein enzyme catalase, which causes the reaction of splitting hydrogen peroxide (H 2 O 2) into oxygen and water, loses its catalytic activity after exposure to high temperature. This is why a drop of hydrogen peroxide applied to a slice of raw potato containing a large amount of catalase “boils”, but on boiled potatoes it remains unchanged.
Enzyme proteins causing the passage of chemical reactions, they themselves remain unchanged; they are sometimes compared to a needle, which connects two pieces of fabric together, while itself remains unchanged.
Enzymes are located in a certain way both in the cell and in the body as a whole. In a cell, enzymes are often associated with its membranes or the membranes of individual organelles (mitochondria, plastids, etc.). The effect of drugs, hormones, and poisons on the body is that they can stimulate or, conversely, inhibit one or another enzymatic process.
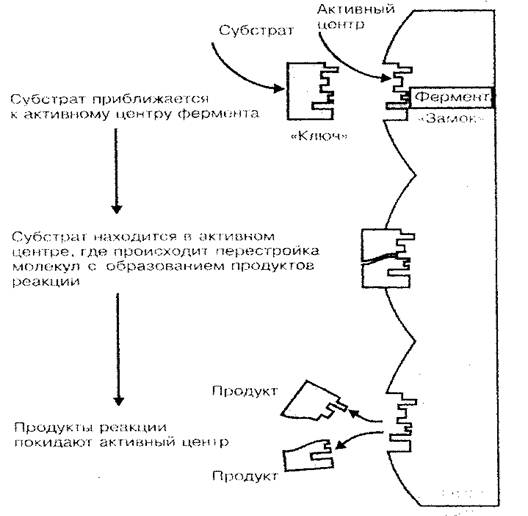
Rice. 3 Scheme of enzyme binding to substrate
Organisms are able to regulate the biosynthesis of enzymes. This makes it possible to maintain the relative constancy of their chemical composition, regardless of constantly changing environmental conditions.
Laboratory work No. 1

